Elimination of Lead Ions from Aqueous Environment by Waste Tires Rubber
Eman Fadhil Nassar , Dheaa Zageer and Majid S. Khlaf *
1Department of Chemistry, College of Science, Al-Nahrain University, Baghdad 64021, Iraq, College of Science, Al-Nahrain University, Baghdad, Iraq .
Corresponding author Email: majidalsaadi@hotmail.com
DOI: http://dx.doi.org/10.13005/OJPS02.02.06
The investigation goes through paper to taking away of lead ions from aqueous medium by waste tire rubber granules(WTRG). The effect of various operational parameters like period of time, original metal concentration, size of adsorbent particles, adsorbent dose, and PH of medium on the adsorption ability of WTRG was evaluated. The kinetic of the adsorption process was relatively fast with approximately one hour of equilibrium time. A tiny adsorbent particle size (0.04 mm), PH medium (5-6) preferential the adsorption process. Data of isotherm studies exposed that adsorption of Pb(II) was well expressed by the Langmuir isotherm formula (R2=0.993). The study demonstrated the ability of WTRG to remove lead from aqueous environment as a cheap and available material. The use of WTRG exhibited the safe disposal of solid waste. Hence its with double benefit for environment.
Copy the following to cite this article:
Nassar E. F, Zageer D, Khlaf M. S. Elimination of Lead Ions from Aqueous Environment by Waste Tires Rubber. Orient J Phys Sciences 2017;2(2).
DOI:http://dx.doi.org/10.13005/OJPS02.02.06Copy the following to cite this URL:
Nassar E. F, Zageer D, Khlaf M. S. Elimination of Lead Ions from Aqueous Environment by Waste Tires Rubber. Orient J Phys Sciences 2017;2(2). Available from: https://bit.ly/3hLU0bu
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 15-12-2017 |
|---|---|
| Accepted: | 30-12-2017 |
Introduction
The existence of heavy metals in the aquatic environment is one of the main concerns due to its toxicity and they tend to bioaccumulation and threat to human life. According to the recommendations of WHO (World Health Organization), the presence of lead in drinking water above the permissible limit (0.015 mg/L) may cause adverse health effects.Hence it is very important that these heavy metals should be detached from discharged wastewater before into an aquatic environment 1,2
The expansion in these lethal heavy metals focus due to fast modern advancement requires constant improvement of the techniques for its expulsion from wastewater. The conventional techniques(such as solvent extraction , precipitation and ion exchange), have various disservices: high costs, low proficiency particularly at low concentration of metal as well as the generation of toxic protectors that require facilitate safe disposal 2
Among all the methods of removing heavy metals, adsorption is an productive technique for water treatment according to the rules of EPA and WHO3.Its more extensive relevance is because of its cost adequacy and environment friendly. The cost adequacy of adsorption innovation is because of the utilization of available adsorbents from Industrial solid waste 4and agricultural wastes5. Accordingly, specialists have concentrated on discovering Low-cost materials suitable as an alternative to activated carbon because of its high cost, for example, bagasse sugar 6starch xanthate 7,sawdust of Pinus sylvestris 8 ,chitosan9, bentonite10, and disposed of vehicle tires11 .
Every year, millions tons of waste tire are produced the world over, which makes the issue of strong waste running significantly more hard to manag. Entire tires fill in as reproducing reason for illness conveying rodents and mosquitoes.. These tremendous quantities of waste tires speak to an enormous natural issue as well as a shabby hotspot for the readiness of adsorbent materials that might be helpful for the evacuation of harmful and danger contaminations from aqueous environment.12,13
Tire rubber is a blend of different elastomers, for example, natural rubber (polyisoprene), styrene-butadiene rubber in addition to different added substances like carbon black, zinc oxide and sulfur. Roughly 32% by waste tire's weight of carbon black constituents in which the carbon content is high (70-75) wt. %. Carbon black is utilized to fortify the tire elastic and to improve its resistance abrasion. This part is very similar to activated carbon in its properties as an adsorbent; the only clear physical difference is that the surface area of activated carbon is less. 14The potentialities of scrap tire in pollutant attenuation in waste streams have been investigated and reported by different researchers. Knocke and Hemphill15have reported the use of scrap tire rubber on Mercury (II) elimination as of aqueous medium , Al-Asheh and Banat 16 have reported the use of scrap tire rubber for heavy metal subtraction from aqueous medium, Jae et al17 studied the sorption of organic materials onto tire rubber. Oladoja et al., 18 also evaluated the potential of scrap tire as an adsorbent for Cu ion and the adsorption process variables (sorbent dosage, pH, time, and original concentration) that define the process.
The purpose of this report is to investigate the potential usage of recycled tire rubber as adsorbent in the elimination of lead ions from aqueous medium. The influence of various parameters on the viability of adsorption have been tested, such as time period, preliminary metal concentration, adsorbent amount, agitation speed and PH of the aqueous medium.
Materials and protocols
Adsorbent (WTRG) Preparation
The waste tires rubber granules (WTRG) used for this study were collected from General Motors Company in Najaf City, Iraq. There was no steel content. Ground rubber of size 0.04 to 0.6 mm was employed in this work. Then, washing the tire granules by distilled water to remove any foreign materials, it was subsequently oven dried at 50- 60°C for 4 h and lay up in airtight containers for following apply.19
Adsorbates Preparation
Lead nitrate salt Pb(NO3)2 was used to prepare the stock solution by dissolving the appropriate amount in de-ionized water. A series of lead ion solutions was prepared (5-80) ppm. The PH of the solution was modified using diluted acid (0.1 M HCl) and base (0.1 M NaOH) solutions.
Batch adsorption experiments
Conducting of batch experiments were done to gain data of lead amount under changeable the initial concentration of lead by crumb rubber, PH, agitation speed, adsorbent particle size and contact time. A certain concentration of lead ions (50 ml) solution was adjusted to a certain PH was transferred to the Erlenmeyer flasks packed with 0.5 g ground crumb rubber and then immersed in a temperature controlled water bath shaker. Specious were taken at desired time intervals, Filter paper (Watman No. 42) was used to filter the samples. Progress in the adsorption process was estimated by measuring the concentration of residual lead ions using the atomic absorption spectrophotometer . All samples were run in duplicate.
The percent removal of Pb2+from aqueous medium was determined using the formula:
Removal % = [C0 - C0)/C0] x 100 (1)
The adsorbed amount of metal is then calculated using the following formula:
qe = V (C0 - Ce)/ W (2)
Co and Ce represent the preliminary and equilibrium concentration of metal (mg/l) in the aqueous medium. V is the volume of the medium (L) and W is the mass of dry adsorbent used (g).qe is the quantity of adsorbed metal per gram of dry adsorbent (mg/g) .
Results and Discussion
Effect of initial pH
The pH represents part of the fundamental contributors within the adsorption of heavy metals ions in aqueous medium due to its impact at the surface charge of adsorbent as well as the speciation of the adsorbate. However, pH has an impact on the ability of adsorption was studied over a variety of pH values from 3.0 to 6.0 so can keep away from precipitation of lead hydroxides, that has been expected to take place at pH >6.5. At pH 3.0 it was observed that the lead ions absorption was low and this could be since the competition for the binding sites of the WTRG sorbent (negatively charged) between Pb(II) ions and H+ or H3o+ ions found in the solution at low pH .The adsorption of Pb(II) will increase whilst pH raises from 3.0 to 6.0 to attain maximum at pH 5–6 where competition between the ions previously mentioned much less. The outcomes are shown in Fig.1.The subsequent experiments were set within the medium of pH 6. The same trend was mentioned by Gupta et al 20.
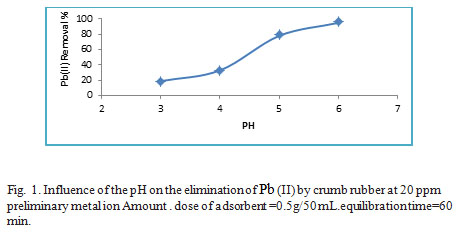 |
Figure 1: Influence of the pH on the elimination of Pb (II) by crumb rubber at 20 ppm preliminary metal ion Amount . dose of adsorbent =0.5g/50 mL.equilibration time=60 min.
|
Impact of time period
Experiments of adsorption as a function of time were conducted and data of time influence on the adsorption capacity of WTRG is presented in Fig.2. It could be clear that there is a rapid adsorption of Pb(II) during the first 60 min. Explanations of this fast uptake by the presence of many active sites were done, but with the passage of time, the active sites get saturated resulting in slower metal removal. It is clear from Fig.2.the equilibrium was obtained approximately at 1 h, that point to the special adsorbent own a quicker rate of adsorption and advanced capacity adsorption for recovery of Pb(II).21
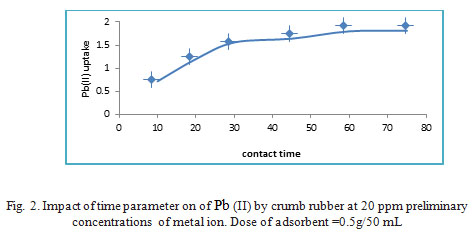 |
Figure 2: Impact of time parameter on of Pb (II) by crumb rubber at 20 ppm preliminary concentrations of metal ion. Dose of adsorbent =0.5g/50 mL
|
Agitation speed role
Impact of the agitation speed on lead ions adsorption on WTRG was focused using different agitation speed from 0 to 160 rpm. Observation of eliminated lead ion percentages were increased while increasing the agitation speed as shown in Fig.3. explanations' of this data through illustrating the mechanism of removing from aqueous medium which involved four stages: The primer stage included migration of lead ions from the bulk medium to the adsorbent surface. The second step involves the diffusion throughout the boundary layer to the adsorbent surface. Third stage engaged adsorption at sites. The fourth stage engaged the diffusion of the intraparticle into the adsorbent interior. Growing the agitation speed could cause decreasing the boundary layer resistance of lead ions transfer from the bulk medium to the surface of adsorbent. However, the adsorbate is compulsory moved towards the surface of adsorbent and guides to an increase in the adsorbate diffusion into the adsorbent surface22,23,24 . The optimized agitation speed selection was to be 130 rpm to supply fine contact among the lead ions in the medium and the solid particles, which confirmed effectively the transfer of metal ions to the adsorbent sites.
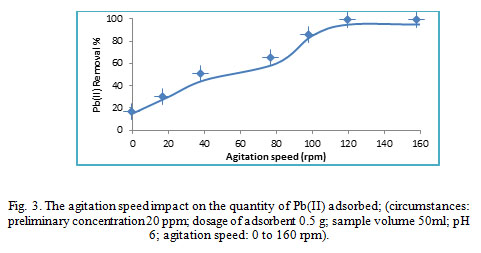 |
Figure 3: The agitation speed impact on the quantity of Pb(II) adsorbed; (circumstances: preliminary concentration 20 ppm; dosage of adsorbent 0.5 g; sample volume 50ml; pH 6; agitation speed: 0 to 160 rpm)
|
Dosage effect
The effect of WTGR dosage (adsorbent mass) on adsorption of lead ions was examined by varying the adsorbent mass from 0.1 to 0.5 g/50ml of solution. From (Fig.4), it has been found that the percentages of adsorbed lead ions were enlarged as the adsorbent dosage was increased. This can be relied on the fact that an increasing in adsorbent dosage boosts up numerically the active sites exist for adsorption25.The elimination approximately 95% was executed when 0.5g was applied.
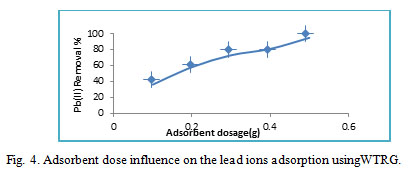 |
Figure 4: Adsorbent dose influence on the lead ions adsorption usingWTRG
|
Adsorbent particle size effect on the adsorption lead ions
Particle size of adsorbents influences adsorption of lead ions. Smaller particle size provide large surface area hence a higher number of surface active sites26. The influence of adsorbent particle sizes on lead ions adsorption was investigated and the metal ions adsorbed with variation in particle sizes for WTRG is shown in Fig.5.
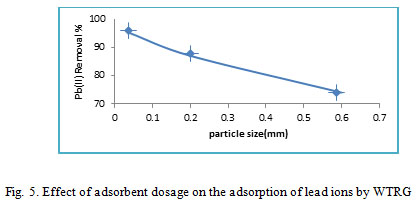 |
Figure 5: Effect of adsorbent dosage on the adsorption of lead ions by WTRG
|
Isotherm studies
In order to describe the equilibrium between the concentration of lead ions in the solution and that in the solid (adsorbent) phase, adsorption isotherms were used. Both Langmuir and Freundlich isotherm formula were employed in this project.
Langmuir isotherm
The general form of the Langmuir formula can be given as follow27:
Ce / qe = (1/klqmax) + (1/qmax)Ce (3)
Ce represents the equilibrium concentration (mg/L), qe is the quantity of lead ions that sorbed, KL is the Langmuir sorption constant (L/mg), qmax is the maximum sorption capacity (mg/g). A linear plot of Ce/qe against Ce is utilized to provide the values of qmax and KL from the slope and the intercept, consecutively (Fig.6). These factors, In addition to the correlation coefficient (R2), of Langmuir formula for the sorption of lead ions by WTRG are written in Table 1.
Table 1: The Langmuir and Freundlich isotherm model constants
|
Langmuir |
Freundlich |
||||
|
qmax(mg/g) |
KL(L/mg) |
R2 |
1/n |
kf(mg/g) |
R2 |
|
8.285 |
0.317 |
0.993 |
0.467 |
2.174 |
0.971 |
Freundlich Isotherm
The Freundlich isotherm is an practical formula used to explain the heterogeneous systems. The Freundlich formula is written as follow28 :
qe = kfc1e (4)
The formula can be written in linear form as:
In qe = Inkf + 1/nIn Ce (5)
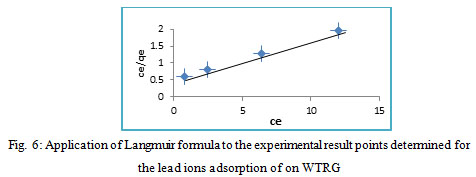 |
Figure 6: Application of Langmuir formula to the experimental result points determined for the lead ions adsorption of on WTRG
|
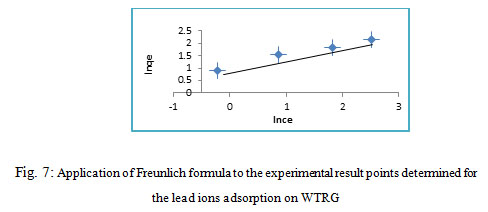 |
Figure 7: Application of Freunlich formula to the experimental result points determined for the lead ions adsorption on WTRG
|
Conclusions
This study examined the elimination of lead ions from aqueous environment using waste tire rubber as an adsorbent. Adsorption process was quick kinetic (60 min) and it was influenced by many parameters, agitation speed of (130 rpm), tiny adsorbent particle size (0.04 mm), PH medium (5-6),and higher adsorbent dose supported the adsorption process. The studies of isotherm show that the adsorption of lead ions was well portrayed by langmuir model. The utilization of WTRG exhibited the safe disposal of solid waste, Hence it's with double benefit to environment.
Acknowledgment
I want to express my thanks and appreciation to the staff of the Department of Chemistry in Colloge of Science / Al-Nahrain University especially of Dr. Emad-Yousif for their help in supporting this project.
References
- Mousavi, H. Z., Hosseynifar, A., Jahed, V. & Dehghani, S. A. M. Removal of lead from aqueous solution using waste tire rubber ash as an adsorbent. Brazilian J. Chem. Eng. 27, 79–87 (2010).
- Grudić, V. V, Brašanac, S., Vukašinović-Pešić, V. L. & Blagojević, N. Z. Sorption of cadmium from water using neutralized red mud and activated neutralized red mud. ARPN J. Eng. Appl. Sci. 8, 933–943 (2006).
- Ali, I. & Gupta, V. K. Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661 (2006).
- Gupta, V. K., Carrott, P. J. M., Ribeiro Carrott, M. M. L. & Suhas. Low-cost adsorbents: growing approach to wastewater treatment-a review. Crit. Rev. Environ. Sci. Technol. 39, 783–842 (2009).
- Akhtar, M., Iqbal, S., Kausar, A., Bhanger, M. I. & Shaheen, M. A. An economically viable method for the removal of selected divalent metal ions from aqueous solutions using activated rice husk. Colloids Surfaces B Biointerfaces 75, 149–155 (2010).
- Gupta, V. K., Jain, C. K., Ali, I., Sharma, M. & Saini, V. K. Removal of cadmium and nickel from wastewater using bagasse fly ash_a sugar industry waste. Water Res. 37, 4038–4044 (2003).
- Wing, R. E., Doane, W. M. & Russell, C. R. Insoluble starch xanthate: use in heavy metal removal. J. Appl. Polym. Sci. 19, 847–854 (1975).
- Taty-Costodes, V. C., Fauduet, H., Porte, C. & Delacroix, A. Removal of Cd (II) and Pb (II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard. Mater. 105, 121–142 (2003).
- Ngah, W. S. W., Ab Ghani, S. & Kamari, A. Adsorption behaviour of Fe (II) and Fe (III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 96, 443–450 (2005).
- Bereket, G., Arog, A. Z. & Özel, M. Z. Removal of Pb (II), Cd (II), Cu (II), and Zn (II) from aqueous solutions by adsorption on bentonite. J. Colloid Interface Sci. 187, 338–343 (1997).
- Rowley, A. G., Husband, F. M. & Cunningham, A. B. Mechanisms of metal adsorption from aqueous solutions by waste tyre rubber. Water Res. 18, 981–984 (1984).
- Hamadi, N. K., Swaminathan, S. & Chen, X. D. Adsorption of paraquat dichloride from aqueous solution by activated carbon derived from used tires. J. Hazard. Mater. 112, 133–141 (2004).
- Alshukri, A. A. et al. Mechanical and Curing Properties of Crumb Rubber Irradiated in Polar Media Filled the Tire Tread Blend. J. Comput. Theor. Nanosci. 14, 5253–5260 (2017).
- Gupta, V. K., Gupta, B., Rastogi, A., Agarwal, S. & Nayak, A. Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Res. 45, 4047–4055 (2011).
- Knocke, W. R. & Hemphill, L. H. Mercury (II) sorption by waste rubber. Water Res. 15, 275–282 (1981).
- Al-Asheh, S. & Banat, F. Adsorption of copper ions on to tyre rubber. Adsorpt. Sci. Technol. 18, 685–700 (2000).
- JY., K. SORPTION OF ORGANIC COMPOUNDS IN THE AQUEOUS PHASE ONTO TIRE RUBBER. J. Environ. Eng. 123, 827–835 (1977).
- Oladoja, N. A., Ofomaja, A. E., Idiaghe, J. A., Akinlabi, A. K. & Egbon, E. E. Sorption of Cu (II) ion from aqueous solution by scrap tyre. Desalin. Water Treat. 16, 83–94 (2010).
- Aisien, F. A., Amenaghawon, N. A. & Akhidenor, S. A. Adsorption of Ethylbenzene from Aqueous Solution Using Recycled Rubber from Scrap Tyre. J. Sci. Res. Reports 2, 497–512 (2013).
- Gupta, V. K., Ganjali, M. R., Nayak, A., Bhushan, B. & Agarwal, S. Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chem. Eng. J. 197, 330–342 (2012).
- Al-Saadi, A. A., Saleh, T. A. & Gupta, V. K. Spectroscopic and computational evaluation of cadmium adsorption using activated carbon produced from rubber tires. J. Mol. Liq. 188, 136–142 (2013).
- Perez-Aguilar, N. V, Diaz-Flores, P. E. & Rangel-Mendez, J. R. The adsorption kinetics of cadmium by three different types of carbon nanotubes. J. Colloid Interface Sci. 364, 279–287 (2011).
- Mane, S. M., Vanjara, A. K. & Sawant, M. R. Removal of phenol from wastewater using date seed carbon. J. Chinese Chem. Soc. 52, 1117–1122 (2005).
- Gupta, V. K., Agarwal, S. & Saleh, T. A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 45, 2207–2212 (2011).
- Hefne, J. A. et al. Kinetic and thermodynamic study of the adsorption of Pb (II) from aqueous solution to the natural and treated bentonite. Int. J. Phys. Sci. 3, 281–288 (2008).
- Aisien, F. A., Amenaghawon, N. A. & Akhidenor, S. A. Adsorption of ethylbenzene from aqueous solution using recycled rubber from scrap tyre. J. Sci. Res. Rep. 2, 497–512 (2013).
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918).
- Freundlich, H. M. F. & others. Over the adsorption in solution. J. Phys. Chem 57, 1100–1107 (1906).

This work is licensed under a Creative Commons Attribution 4.0 International License.





