A Study of Ethanol Content and Octane Effects in Ethanol Mixed Petrol
S. A Iqbal Crescent College of Technology, Karodh, M.P, Bhopal. , R K Sharma Central Lab ‘K’ Oil Installation, Sawree, Mumbai. * , Subrata Bhattacharjee Central Lab ‘K’ Oil Installation, Sawree, Mumbai. and M J Shaikh Central Lab ‘K’ Oil Installation, Sawree, Mumbai.
Corresponding author Email: yajkumarsharma@indianoil.in
A fuel with a higher octane rating can be run at a higher compression ratio without causing detonation. Compression is directly related to power and to thermodynamic efficiency (see engine tuning), so engines that require higher octane usually deliver more motive power and do more work for a given BTU or calorie of fuel. Engine power is a function of the fuel, as well as the engine design, and is related to octane rating of the fuel.
Copy the following to cite this article:
Iqbal S. A, Sharma R. K, Bhattacharjee S, Shaikh M. J. A Study of Ethanol Content and Octane Effects in Ethanol Mixed Petrol. Orient J Phys Sciences 2017;2(1).
Copy the following to cite this URL:
Iqbal S. A, Sharma R. K, Bhattacharjee S, Shaikh M. J. A Study of Ethanol Content and Octane Effects in Ethanol Mixed Petrol. Orient J Phys Sciences 2017;2(1). Available from: http://orient
Download article (pdf) Citation Manager
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Introduction
Octane The quality of various petroleum fuels depends on their composition and types of hydrocarbons present in the mixture. Octane number is one of the characteristics of spark –ignition engine fuel such as gasoline. This number indicates anti knocking characteristics of a fuel and strongly depends on the hydrocarbon type. Octane number of fuels can be improved by addition of oxygenates such as TEL, MTBE or TAME.Oxygenated gasoline is not new; oxygenates have been added to gasoline for decades. Prior to 2006, methyl tertiary butyl ether (MTBE) and tertiary amyl methyl ether (TAME) along with smaller quantities of ethyl tertiary butyl ether (ETBE) were used in U.S. gasoline.
Today, ethanol, also called ethyl alcohol or grain alcohol, is used almost exclusively.
Widespread use of ethanol in the United States began in 1978 when a Nebraska group marketed gasoline containing 10 volume percent ethanol as gasohol. The program was intended to help increase gasoline availability during the energy crisis of that decade.
Later, the gasohol name was abandoned, but the use of ethanol continued. Much of the early ethanol-blended product was marketed in the Midwestern states, where the bulk of ethanol is produced.
In the 1990s, air quality regulations required gasolines in some parts of the United States to be oxygenated either in the winter or else year-round. By 2001, about 10 percent of all the gasoline sold in the U.S. contained ethanol and 58 percent of the blends contained 10 volume percent ethanol, often referred to as E10. Initially, little RFG contained ethanol.
MTBE was used as an oxygenate to meet U.S. Environmental Protection Agency (EPA) and California Air Resources Board (CARB) reformulation requirements. The first U.S. EPA waiver for MTBE use was issued in 1979 for 7.0 volume percent, and a second waiver for 15.0 volume percent was issued in 1988. MTBE was not widely used until the late 1980s, and then its use expanded with the introduction of federal RFG in 1995. California banned the use of MTBE and other ethers and heavy alcohols as of January 1, 2004. A number of other states followed California’s lead, which greatly diminished MTBE use. In 2006, the U.S. EPA rescinded the oxygenated gasoline requirement in federal RFG, as required by the Energy Policy Act of 2005. This resulted in the removal of virtually all MTBE from the U.S. gasoline pool. Gasoline refiners in the U.S. ceased using MTBE, and by default, ethanol became the oxygenate component used almost exclusively.
Chemistry
Oxygenated gasoline is a mixture of conventional hydrocarbon-based gasoline and one or more oxygenates. Oxygenates are combustible liquids made up of carbon, hydrogen, and oxygen. All the oxygenates currently used in gasoline belong to one of two classes of organic molecules: alcohols and ethers. In alcohols, a hydrocarbon group and a hydrogen atom are bonded to an oxygen atom: R-O-H, where R represents the hydro-carbon group. All alcohols contain the OH atom pair. In ethers, two hydrocarbon groups are bonded to an oxygen atom; the groups may be the same or different: R-O-R or R-O-R’ (the apostrophe is used to indicate a different hydrocarbon group).
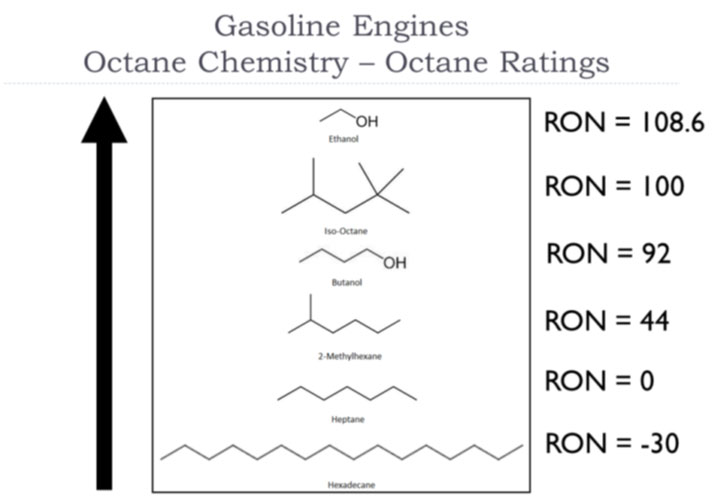 |
Figure 1
|
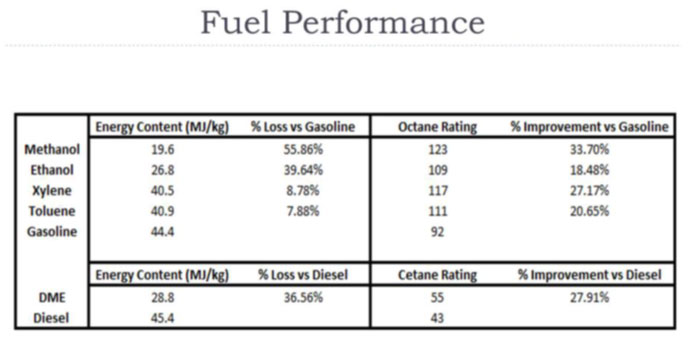 |
Figure 2
|
History of Octane Ratings
In 1921, the Co-operative Fuel Research Committee (CFRC) was formed to research fuels and engine performance. The CFR engine was developed to evaluate knock performance of fuels under controlled conditions, measuring knock intensity with a bouncing pin knockmeter. In 1927 Graham Edgar developed an octane rating scale based on high and low reference fuels, and showed that fuels had Octane Ratings between 40-60. This became the C.F.R. Research Octane Number in 1930. The RON test method was first published in 1932, became a tentative method in 1947, and was adopted as an ASTM standard in 1951.
Graham Edgar used two pure hydrocarbon isomers that could be produced in sufficient purity and quantity as reference fuels for rating the anti-knock ability of fuel. These were "normal heptane," which was commercially available in sufficient purity from the distillation of Jeffrey pine oil, and "an octane, named 2,4,4-trimethylpentane" that he first synthesized (commonly Ccdled isooctane today, although there are 17 different isomers possible, and about five prevalent C8 isomers from C4 alkylation). The heptane and iso-octane were given the arbitrary octane numbers of 0 and 100, respectively.
These have similar volatility properties (Table 1), specifically boiling point, thus the varying ratios 0:100 to 100:0 would have consistent vaporization behavior. The volume percentage of isooctane in the binary blend that gave the same knock intensity as the fuel being tested was assigned as the Octane Number of the sample.
The early work quickly identified that the "highest useful compression ratio" (efficiency, power) was related to the CFR Research octane number. The sulfur-containing gasoline was initially restricted because sulfur in gasoline inhibited the octane- enhancing effect of the alkyl lead. In addition, different
Table 1: Selected properties of normal heptane and iso-octane.
|
|
Melting Point, °C |
Boiling Point, "C |
Density, g/ml |
Heat of Vaporization, MJ/kg
|
|
Normal heptane
|
-90.7 |
98.4 |
0.684 |
0.365 at 25°C
|
|
Iso octane
|
-107.45 |
99.3 |
0.6919 |
0.308 at 25°C
|
alkyl leads. Parafins have the best alkyl lead response, followed by napthenes, olefins, and aromatics, while the response of alcohols is negative. The RON of the gasoline could be increased from 60 to 75, and the "highest useful compression ratio" increased from 5.3 to 6.8, increasing the constant speed fuel economy by about 30%.
The Motor Octane Number (MON) was developed in 1932, using conditions that better matched actual vehicle knock performance climbing the long constant grade hill at Uniontown, Pennsylvania. This method is similar to the operating conditions of the current Motor Octane procedure. The MON test was a tentative method from 1933 to 1939, when it was adopted as an ASTM standard. During the late 1940s through the middle 1960s, the Research method became the more important rating because milder conditions more closely represented the octane requirements of the vehicle current engines and driving conditions. Most retail fuels were marketed according to their research octane rating.
1963 Clean Air Act, V e h i c l e Emission Standards and Alkyl l e a d Phase down
During the late 1950s and 1960s, ambient air quality deteriorated, especially in densely populated urban areas. ReseEirch quickly identified the "primary pollutants" in raw exhaust (HC, CO, and NOx) and sunlight driven photochemical reaction to be a major source of SMOG (SMoke + fOG). Ground level ozone and other "secondary poUutcints" are formed by photochemical reactions driven by sunlight. The concentrations of pollutants followed a daily pattern determined by driving cycles ("rush hours"), daytime sunlight intensity, and local geography and wind patterns, with the most severe area in the U.S. being southern California. The original Clean Air Act was passed in 1963 "to protect and enhance the quality of the Nation's air resources." The first vehicle emissions standards were imposed in California in 1966 and Federally in 1968. The early attempts to reduce HC and CO with carburetor calibrations and thermal reactor ("thermactor," air + time delay) type exhaust systems were only about 60% effective for HC. However, this was still a larger drop (est. 10.6 g/mile to 4.1 g/mile HC) than all of the emission reductions since, due to the high emission rates at the time. A number of attempts were made to develop "lead-tolerant catalysts" and "alkyl lead traps" that would allow continued use of leaded gasoline. However, several new potential problems with continued widespread use of leaded gasoline were found, and R&D to develop viable exhaust oxidation catalysts went into high gear.
Alkyl lead is a toxin by itself, and there was a drive to reduce exposure levels from many sources, ranging from alkyl lead solder in pipes, food cans, toothpaste tubes, alkyl lead pigment in paints, and edkyl leads in gasoline. In addition, the alkyl lead scavengers ethyene dibromide and ethylene dichloride could react with unbumed hydrocarbons in the exhaust to form traces of organo-halogen compounds, including dioxins. It is unlikely that high levels of TEL use would have continued even if a lead-tolerant catalyst had been found.
Separate regulations were put in place to minimize the amount of alkyl lead (primeirily TEL) used in the remaining leaded gasoline produced and sold, creating "low lead" in a series of reductions in 1985/86. A smaller fill nozzle diameter was adopted for nonleaded gasoline to prevent misfueling catalyst cars with leaded gasoline. In the United States, alkyl lead could not be knowingly added to nonleaded gasoline, and the maximum allowable concentration of 0.05 g/usgal (13 rng/L) was applied as a contamination limit for "incidental contact" with leaded fuels during distribution. Other jurisdictions have slightly different legal definitions and contamination limits for nonleaded gasoline, but the terms "nonleaded" and "unleaded" tend to be used interchangeably, even if this is not technically or legally correct in all cases.
Octane ratings of leaded gasoline decreased from the "octane race" levels, about 1.5-3 AKI lower for RUL and PUL, respectively.
Some predominately smEJl engines were designed for 87 AKI nonleaded fuel, but were "alkyl lead tolerant," because they could meet the emission standards of the time without a catalytic converter, so could use either leaded or nonleaded
gasoline. The last premium leaded gasoline was sold in 1981, and the last regular leaded in 1996 in the United States, and in 1990 in Canada.
Alkyl alkyl lead has been credited variously with allowing rapid development of high power Eind efficiency SI engines, and even being a deciding factor in aviation in World War II. It is not widely appreciated that Thomas Midgley's other famous discovery, perfluorocarbon refrigerEint, had the same dichotomous history as alkyl lead. It provided enormous social benefits, but was eventually banned due to stratospheric ozone depletion. It was replaced with less long-lived halocarbons that are decomposed at lower altitudes.
How to measure Octane rating ?
The octane number of a fuel is measured in a test engine, and is defined by comparison with the mixture of 2,2,4-trimethylpentane (iso-octane) and heptane which would have the same anti-knocking capacity as the fuel under test: the percentage, by volume, of 2,2,4-trimethylpentane in that mixture is the octane number of the fuel.
For example, petrol with the same knocking characteristics as a mixture of 90% iso-octane and 10% heptane would have an octane rating of 90. This does not mean that the petrol contains just iso-octane and heptane in these proportions, but that it has the same detonation resistance properties. Because some fuels are more knock-resistant than iso-octane, the definition has been extended to allow for octane numbers higher than 100.
Octane rating does not relate to the energy content of the fuel. It is only a measure of the fuel's tendency to burn in a controlled manner, rather than exploding in an uncontrolled manner. Where the octane number is raised by blending in ethanol, energy content per volume is reduced.
It is possible for a fuel to have a Research Octane Number (RON) greater than 100, because iso-octane is not the most knock-resistant substance available.
Racing fuels, avgas, liquefied petroleum gas (LPG), and alcohol fuels such as methanol may have octane ratings of 110 or significantly higher. Typical "octane booster" gasoline additives include MTBE, ETBE, isooctane and toluene.
Lead in the form of tetra-ethyl lead was once a common additive, but since the 1970s, its use in most of the industrialised world has been restricted, and its use is currently limited mostly to aviation gasoline.
Effects of octane rating
Higher octane ratings correlate to higher activation energies. Activation energy is the amount of energy necessary to start a chemical reaction. Since higher octane fuels have higher activation energies, it is less likely that a given compression will cause auto ignition.
It might seem odd that fuels with higher octane ratings are used in more powerful engines, since such fuels ignite less easily. However, an uncontrolled ignition is not desired in a spark ignition engine.
A fuel with a higher octane rating can be run at a higher compression ratio without causing detonation. Compression is directly related to power and to thermodynamic efficiency (see engine tuning), so engines that require higher octane usually deliver more motive power and do more work for a given BTU or calorie of fuel. Engine power is a function of the fuel, as well as the engine design, and is related to octane rating of the fuel. Power is limited by the maximum amount of fuel-air mixture that can be forced into the combustion chamber. When the throttle is partially open, only a small fraction of the total available power is produced because the manifold is operating at pressures far below atmospheric. In this case, the octane requirement is far lower than when the throttle is opened fully and the manifold pressure increases to atmospheric pressure, or higher in the case of supercharged or turbocharged engines.
Many high-performance engines are designed to operate with a high maximum compression, and thus demand high-octane premium gasoline. A common misconception is that power output or fuel mileage can be improved by burning higher octane fuel than specified by the engine manufacturer. The power output of an engine depends in part on the energy density of its fuel, but similar fuels with different octane ratings have similar density. Because switching to a higher octane fuel does not add more hydrocarbon content or oxygen, the engine cannot produce more power.
However, burning fuel with a lower octane rating than required by the engine often reduces power output and efficiency one way or another. If the engine begins to detonate, that reduces power and efficiency for the reasons stated above. Many modern car engines feature a knock sensor – a small piezoelectric microphone which detects knock, and then sends a signal to the engine control unit to retard the ignition timing. Retarding the ignition timing reduces the tendency to detonate, but also reduces power output and fuel efficiency. Because of these systems, under certain conditions of high load and high temperature, a given car may produce more power with a higher octane fuel. With a lower octane fuel, these engines systems will be reducing power to control detonation, while with a higher octane fuel, the engine will produce full power. And some modern high performance engines are actually optimized for higher than pump premium (93 AKI in the US). The 2001 - 2007 BMW M3 with the S54 engine is one such car. Car and Driver magazine dyno tested a car and found that the power output increased as the AKI was increased up to approximately 96 AKI. Also, these systems can result in higher fuel mileage for cars designed for the higher octane fuels.
Most fuel stations have two storage tanks (even those offering 3 or 4 octane levels), and you are given a mixture of the higher and lower octane fuel for the intermediate grades. Premium is fuel from the higher octane tank, and the minimum grade sold is fuel from the lower octane tank. Purchasing 91 (where offered) simply means more fuel from the higher octane tank than purchasing 89; the detergents in the fuel are often identical. But for some producers, the additive package is different between the higher and lower octane rating.
The octane rating was developed by chemist Russell Marker at the Ethyl Corporation c1926. The selection of n-heptane as the zero point of the scale was due to the availability of very high purity n-heptane, not mixed with other isomers of heptane or octane, distilled from the resin of the Jeffrey Pine. Other sources of heptane produced from crude oil contain a mixture of different isomers with greatly differing ratings, which would not give a precise zero point
Experiments
Ethanol has a high octane rating and can be added to gasoline to produce high octane fuel blends. Understanding the octane increase with ethanol blending is of great fundamental and practical importance. Potential issues with fuel flow rate and fuel vaporization have led to questions of the accuracy of octane measurements for ethanol-gasoline blends with moderate to high ethanol content (e.g., E10-E30) using the Cooperative Fuel Research (CFR™) engine. The nonlinearity of octane ratings with volumetric ethanol content makes it difficult to assess the accuracy of such measurements. In the present study, Research Octane Number (RON) and Motor Octane Number (MON) were measured for a matrix of ethanol-gasoline blends spanning a wide range of ethanol content (E0, E10, E15, E20, E30) in a set of gasoline blend stocks spanning a range of RON values ( 88, 89, 91, and 98). Octane ratings for neat ethanol, denatured ethanol, and hydrous ethanol were also measured. One set of measurements was conducted using a CFR™ engine equipped with manufacturer-supplied enhancements (GE Energy Waukesha XCP-OA™ digital octane panel) for digital knock measurement and precise control of temperatures and fuel flow. A second set of measurements was conducted at a separate laboratory with a CFR™ engine equipped with an adjustable-orifice fuel jet. Both approaches address fuel flow issues at high ethanol concentrations.
A linear molar octane blending model was found to describe most of the nonlinearity in the RON and MON data, but measured values were still somewhat greater than predicted.
This study supports a companion paper (SAE 2012-01-1277) in which a state-of-the-art single-cylinder engine equipped with multiple fuel injection systems was used to evaluate the knock-limited performance of the ethanol-gasoline blends described herein and to evaluate the relevance of octane ratings and heat of vaporization as predictors of this performance.
The following blends are prepared and tested i.e
Data
|
Sr. No. |
Parameter |
Method |
Pure |
10%E |
15%E |
20%E |
30%E |
|
1.A |
Density @15'C (Manual) |
P-16 |
756.3 |
760.2 |
762.2 |
764.2 |
768.1 |
|
1.B |
RON |
D-2699 |
98.1 |
99.9 |
101 |
101.7 |
101.6 |
|
|
MON |
D-2699 |
86.4 |
87.6 |
88.4 |
88.9 |
89.5 |
|
|
R+M/2 |
D-2699 |
92.3 |
93.8 |
94.7 |
95.3 |
95.6 |
|
2.A |
Density @15'C (Manual) |
P-16 |
745.8 |
749.2 |
751.3 |
753.6 |
757.3 |
|
2.B |
RON |
D-2699 |
88.2 |
92.9 |
94.7 |
96.6 |
99.3 |
|
|
MON |
D-2699 |
80.4 |
83 |
83.8 |
84.6 |
87.9 |
|
|
R+M/2 |
D-2699 |
84.3 |
88 |
89.3 |
90.6 |
93.6 |
|
3.A |
Density @15'C (Manual) |
P-16 |
742.6 |
745.8 |
747.8 |
749.8 |
753.6 |
|
3.B |
RON |
D-2699 |
91.5 |
95.3 |
97.0 |
98.0 |
99.8 |
|
|
MON |
D-2699 |
82.3 |
85.3 |
85.5 |
86.3 |
87.8 |
|
|
R+M/2 |
D-2699 |
86.9 |
90.3 |
91.2 |
92.2 |
93.8 |
|
4.A |
Density @15'C (Manual) |
P-16 |
753.7 |
756.9 |
758.8 |
761.1 |
764.5 |
|
4.B |
RON |
D-2699 |
97.9 |
99.1 |
100 |
100.8 |
101.6 |
|
|
MON |
D-2699 |
86 |
86.9 |
88.3 |
88.3 |
88.1 |
|
|
R+M/2 |
D-2699 |
92 |
93 |
94.2 |
94.6 |
94.8 |
|
5.A |
Density @15'C (Manual) |
P-16 |
737.6 |
740.0 |
741.7 |
744.0 |
747.7 |
|
5.B |
RON |
D-2699 |
89.0 |
93.4 |
95.7 |
97.3 |
99.9 |
|
|
MON |
D-2699 |
81.5 |
83.8 |
84.6 |
85.6 |
88.1 |
|
|
R+M/2 |
D-2699 |
85.3 |
88.6 |
90.1 |
91.5 |
94.0 |
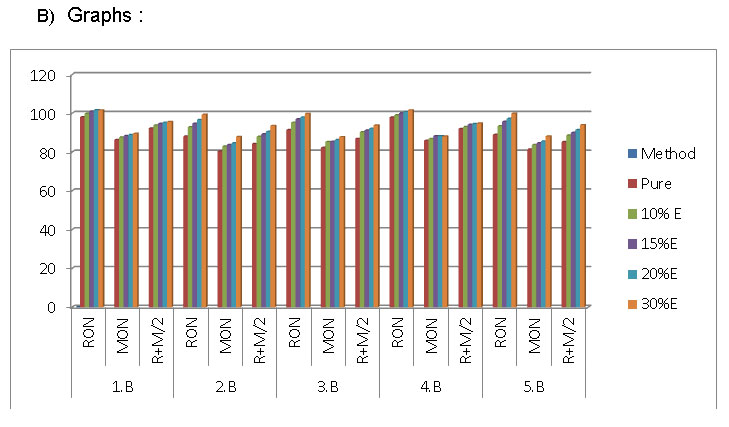 |
Figure 3
|
Discussion
From the graph, both the parameters ( RON & MON) are increasing trend which is having very good sign in ethanol blended petrol.
Gasoline with a higher octane rating is more resistant to engine knock. This is the primary characteristic that distinguishes various grades of gasoline (e.g. regular vs. premium). A gasoline’s octane rating is a function of the chemical composition of the blend. Oxygenates such as ethanol and MtBE are valuable blend components due to their high octane, anti-knock characteristics. In fact, MtBE was first blended in gasoline as an octane enhancer when lead was phases-out. Both MtBE and ethnol are less toxic than many other high-octane components of gasoline, such as benzene.
While ethanol has a slightly higher octane rating than MtBE, it also has substantially higher oxygen content; ethanol at 5.7 percent by volume provides the same by-weight oxygen content as MtBE at 11 percent by volume. Consequently, if blended at the RFG minimum, ethanol is less effective at displacing and diluting other toxic octane-enhancing constituents and may result in a moderate increase in toxic air emissions absent regulatory action. Further, because less ethanol is needed per gallon of RFG, other high-octane constituents will be needed to make up for the octane loss associated with removing MtBE.
Toxic aromatic compounds are the most likely high-octane replacement, which creates the potential for air toxic emission increases during the transition from MtBE to ethanol. High-octane compounds such as benzene and toluene are also a threat to water resources when spilled or leaked into the environment. Therefore, reformulation decisions that affect the amount of these compounds in gasoline also present water quality-related health and environmental implications.
Benefit : Ethanol acts like octane booster
A study done ethanol content ( % by Vol.) in 10% EPMS.
Purpose of this study needs to focus the handling of ethanol mixing in petrol and proper determination in line with latest specification IS 2796 :2014 ( ANNEX E). Looking into the practical facts, samples have been collected from Tank truck around 50 samples in GOA region ( GOA is nearer to SEA and humidity in AIR) and 20 samples from different batches are taken and mixed with Ethanol ( 10% by volume) at Surat laboratory and tested for Ethanol content. As latest specification , Ethanol content in Petrol would be 9.75 +/- 0.25 ( ASTM D 4815/D5599).
Mythology of samples have been taken from Tank truck and make a lab. blend with different batches which are as follows ;
|
S.No. |
Lab. Blends |
Sample taken from Tank Trucks after loading on line |
|
1. |
20 |
50 |
Lab. blend Samples tested at laboratory : Total 20 numbers of samples have been taken from different sources and mixed with 10% ethanol by volumetric and tested.
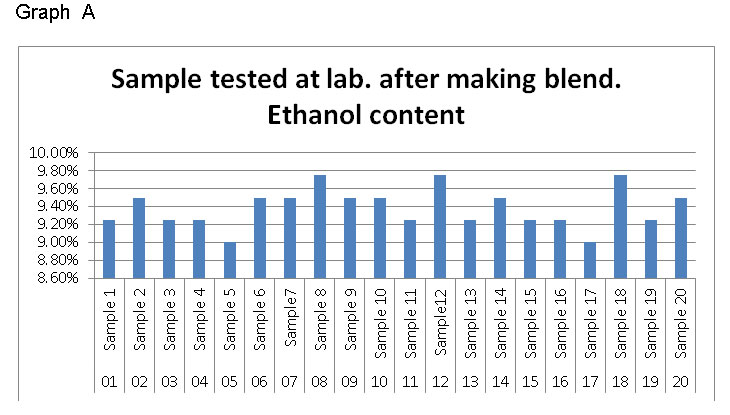 |
Figure 4
|
Samples taken from TTs : Approximate 50 samples taken from TTs after loading which has been mixed on line and tested for ethanol content. Results are shown in graph B.
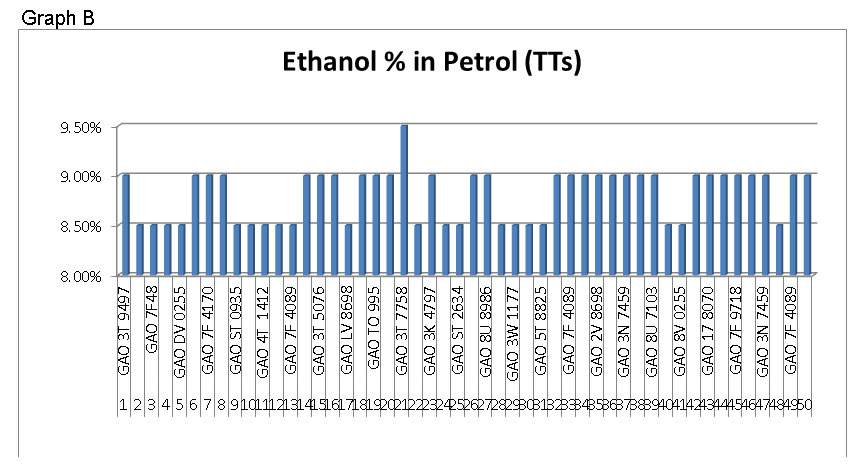 |
Figure 5
|
Outcome
In Ideal condition, only 10 samples are found within the limit; however 10 samples are outside the limits.
In Field condition, it is observed that only one sample is in line with specification parameter; however 29 samples showed just 9 % ethanol content and 20 samples indicated only 8.5% ethanol content in petrol. The variation between specified required against observed is nearly 1.0% by volume which needs to rethink.
Recommendation
Parameter of ethanol content in petrol needs to be changed in 10% by vol. ethanol in petrol Specification for which more data are to be generated.
References
-
- Kemp, Kenneth W.; Brown, Theodore; Nelson, John D. (2003). Chemistry: the central science. Englewood Cliffs, N.J: Prentice Hall. pp. 992.
- Johnson Operation and Maintenance Manual, 1999.
- A. T. Balaban, L. B. Kier, and N. Josh, MATCH (Commun. Math. Chem.) 28 (1992) 13–27.
- Impact of alcohol–gasoline fuel blends on the performance and combustion characteristics of an SI engine,Fuel 89 (2010) 2713–2720.
- Latest BIS Specification IS :2796 :2014 ( 10% EPMS)
- Ingersoll, John G. (1996). Natural gas vehicles. Lilburn, Ga: Fairmont Press. pp. 327.
- Gallagher, P., M. Dikeman, J. Fritz, E. Wailes, W. Gauthier, and H. Shapouri, Biomass from Crop Residues: Some Cost and Supply Estimates, Agricultural Economic Report Number 819, U.S. Dept of Agriculture, January 2003.
- Gallagher, P. and H. Shapouri, “Improving Sustainability of the Corn-Ethanol Industry,” in Biofuels, W. Soetaert and E. Vandamme, eds, John Wiley, West Sussex (UK), December 2008.
- Kwiatkowski, Jason, McAloon, Andrew, Taylor, Frank, Johnston, David; “Modeling the Process and Costs of Fuel Ethanol by the Dry-Grind Process, Industrial Crops and Products 23(2006),” pp. 288-296.
- Pimentel, David, “Ethanol Fuels: Energy Security, Economics, and the Environment,” Journal of Agricultural and Environmental Ethics 4(1991):1-13.
- Shapouri, Hosein, James A. Duffield, and Michael Wang, “The Energy Balance of Corn Ethanol: An Update,” U.S. Department of Agriculture, Office of the Chief Economist, Office of Energy Policy and New Uses, Report No. 813(July 2002).
- Shapouri, H., and P. Gallagher, USDA’s 2002 Ethanol Cost-of-Production Survey, U.S. Department of Agriculture, Office of Energy Policy and New Uses, Agricultural Economic Report No. 841(July 2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.





